WSW, NY, December 4th, 2025, FinanceWire
With antibiotic resistance rising in recent years, the Pentagon has quietly provided funding to a microcap biotech firm whose civilian wound program may also solve one of modern warfare’s most dangerous medical problems
For decades, antibiotics functioned as a silent guardian of modern warfare. After penicillin’s mass production in World War II, battlefield infections – historically a leading cause of death from wounds – fell dramatically with the mass deployment of penicillin and modern surgical care. Today’s soldiers survive injuries that would have been fatal for every generation before them. But that advantage is eroding.
In conflict zones from Eastern Europe to the Middle East, military clinicians are reporting a troubling resurgence of drug-resistant bacteria. Wounds that should heal are instead becoming entrenched infections, often impervious to even the strongest antibiotics. It is a quiet crisis, but one U.S. defense health officials increasingly frame as a threat to force readiness.
What’s interesting is where the U.S. military seems to believe part of the solution may come from: a publicly traded microcap focused on diabetic foot infections. That company is BiomX (NYSE: PHGE) – and despite a market cap hovering at just around to ~$7 million, it has already received approximately $40 million in non-dilutive funding from the U.S. Defense Health Agency and the Department of the Navy under an award managed by the Naval Medical Research Command.
The reason doesn’t seem to be found in its stock chart, but rather in the biology.
Why the Pentagon Cares About a Diabetic Infection Company
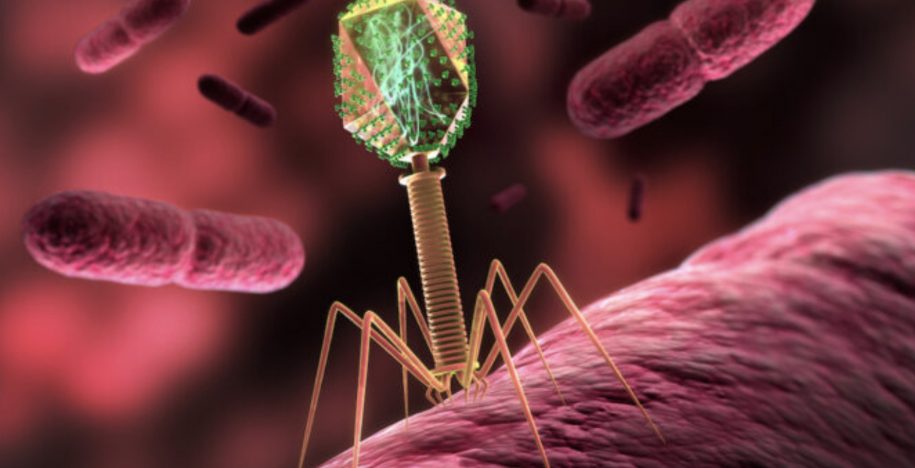
Whether the wound begins with shrapnel or with a diabetic foot ulcer, the same biological enemy often stands in the way of healing: bacteria such as Staphylococcus aureus protected inside biofilms.
Biofilms act like fortified shelters. Once bacteria establish these structures deep inside tissue, antibiotics struggle to penetrate. The immune system can’t reach them. Infection lingers. Tissue dies. Amputation risk rises.
This is why both a soldier’s blast injury and a civilian diabetic ulcer can lead to the same devastating outcome: a limb that cannot be saved.
BiomX develops bacteriophage therapies – precision viruses engineered to target and destroy harmful bacteria, including those hiding in biofilms. Phages replicate at the site of infection and, in preclinical and clinical studies, have shown activity against bacteria within biofilms.
That capability is what brought the military to BiomX’s door.
The Pentagon cannot run clinical trials on an active battlefield. But it can study the same biology in civilian patients – and diabetic foot infections happen to be one of the most predictable, high-volume environments to evaluate phage effectiveness against Staph aureus.
BiomX’s program, therefore, seems to serve two strategic purposes: a legitimate civilian medical need and a proxy model for battlefield trauma care.
Where Civilian Data Meets Military Relevance
BiomX already has initial human data showing that its phage technology can meaningfully impact hard-to-treat infections. In its Phase 2 trial in diabetic foot osteomyelitis – one of the most stubborn chronic infections – the company demonstrated statistically significant improvements in ulcer size (percent area reduction), ulcer depth (in a predefined subgroup), and in limiting ulcer area expansion compared to placebo.
For the Defense Health Agency, these results are likely relevant because they involve deep, biofilm-associated infections where antibiotic resistance is a growing concern — similar to the wound infections seen in combat casualties.
Building on that data, BiomX is now advancing BX011, a topical phage cocktail for diabetic foot infections that occur earlier and more commonly than bone infections. The FDA recently provided feedback confirming that BiomX does not need additional non-clinical studies before proceeding – an important step that gives the program a clearer clinical path.
And here is where the civilian-military link becomes clear: If BX011 proves effective in early-stage diabetic infections, it establishes the same biological principle that military clinicians are urgently looking for in battlefield trauma care. At that point, the Pentagon doesn’t need to reinvent anything – it could potentially adopt an FDA-approved product that has already been validated in civilian care.
It’s a rare example of civilian medicine directly accelerating national defense preparedness.
A Microcap With Outsized Strategic Weight
What makes BiomX notable is not hype – it’s what seems to be a stark potential mismatch between its low market cap and the level of attention it has attracted from U.S. defense and regulatory agencies. The company trades at a market cap of under $10 million as of now, yet it is engaged with the Defense Health Agency and the FDA at a depth typically associated with mid-stage companies worth many times more. The Defense Health Agency routinely funds high-risk research, but securing roughly $40 million in non-dilutive support seems unusual for a company of BiomX’s size and functions as a meaningful vote of confidence in the underlying science.
At the same time, the civilian market BiomX is targeting is enormous. Diabetic foot infections contribute to an estimated 160,000 lower-limb amputations among U.S. diabetic patients every year, creating a multi-billion-dollar annual economic burden. Some analyses estimate that the direct costs of diabetic foot complications alone exceed $8 billion annually. The clinical toll is equally severe: five-year mortality rates following diabetic foot–related amputation are comparable to, and in some cases worse than, several common cancers.
Despite this scale, innovation has been limited. Standard care still relies heavily on long courses of broad-spectrum antibiotics and surgical intervention, even as resistance rises and outcomes stagnate. And while early-stage companies naturally face risks – from cash runway to clinical execution to regulatory outcomes – a technology that can reliably improve outcomes in these infections, especially those driven by drug-resistant bacteria, would not only meet a pressing medical need but could reshape a multi-billion-dollar segment of infectious-disease care.
Recent News Highlights From BiomX
BiomX Reports Third Quarter 2025 Financial Results and Provides Program Updates
BiomX Provides Update on BX004 Phase 2b Trial in Cystic Fibrosis
Important Disclaimers and Disclosures: The author, Wall Street Wire, is a content and media technology platform that connects the market with under-the-radar companies. The platform operates a network of industry-focused media channels spanning finance, biopharma, cyber, AI, and additional sectors, delivering insights on both broader market developments and emerging or overlooked companies. The content above is a form of paid promotional content and advertising. Wall Street Wire receives cash compensation from BiomX Inc for promotional media services provided on an ongoing subscription basis. This content is for informational purposes only and does not constitute financial or investment advice. Wall Street Wire is not a broker-dealer or investment adviser. Full compensation details, information about the operator of Wall Street Wire, and the complete set of disclaimers and disclosures applicable to this content are available at: wallstwire.ai/disclosures. Market size figures or other estimates referenced in this article are quoted from publicly available sources; we do not independently verify or endorse them, and additional figures or estimates may exist. This article should not be considered an official communication of the issuer.