WSW, NY, January 2nd, 2026, FinanceWire
MediWound’s (NASDAQ: MDWD) NexoBrid deployed at Lausanne and Geneva hospitals treating victims of one of Switzerland’s worst disasters
When fire tore through Le Constellation bar in Crans-Montana shortly after midnight on New Year’s Eve, what began as a celebration became one of Switzerland’s deadliest disasters in modern history. According to the latest reports, approximately 45 people were killed and 115 injured, with roughly 50 suffering severe burns that overwhelmed the country’s specialized burn units within hours.
The scale of the emergency forced Swiss authorities to activate a massive response: 150 personnel, 10 helicopters, and 40 ambulances rushed to the alpine resort. Burn victims were airlifted to specialized centers in Lausanne, Zurich, Geneva, and Bern, with some transferred to hospitals in Germany, Italy, and France as Swiss capacity was exhausted.
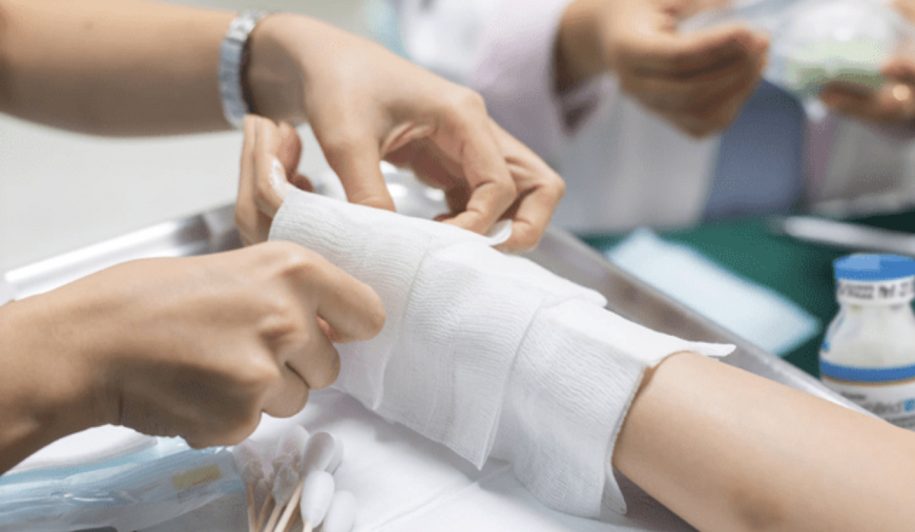
At Lausanne University Hospital (CHUV) and Geneva University Hospitals (HUG), medical teams turned to NexoBrid, an innovative enzymatic burn debridement therapy developed by MediWound Ltd. (NASDAQ: MDWD). According to reports and recent posts, NexoBrid was used in the care of burn patients at these specialized centers, and in select cases continued to be used as patients were transferred to hospitals outside Switzerland, including facilities in Italy, due to capacity constraints. Treatment efforts were supported through MediWound’s Swiss distributor, Triskel Integrated Services.
A Growing Track Record in Mass Casualty Events
The Swiss deployment is not the first time NexoBrid has been called upon during a crisis. In March 2025, a nightclub fire in Kočani, North Macedonia, killed 63 people and injured 193. With burn victims requiring evacuation to hospitals across more than ten countries, Israel dispatched two medical delegations, one from Sheba Medical Center and another coordinated through the Foreign Ministry, carrying NexoBrid to support treatment efforts on-site.
These incidents reflect a broader pattern that has driven governments to stockpile enzymatic burn treatments. NexoBrid uses proteolytic enzymes derived from pineapple stems to perform debridement, the critical process of removing dead or damaged tissue from burn wounds so healthy skin can heal. Traditionally, debridement requires surgery, but NexoBrid accomplishes this in a single four-hour topical application. In clinical trials, complete debridement took an average of one day with NexoBrid compared to six days with standard surgical care, and only 1.5% of wound area required surgical removal versus 48% with standard treatment.
For hospitals managing dozens of severe burn cases simultaneously, this distinction matters. Surgical debridement requires operating room time, anesthesia, and surgical teams, resources that become acutely constrained during mass casualty events.
In contrast, NexoBrid’s enzymatic debridement does not require operating rooms or surgical teams. NexoBrid is applied topically at the bedside, allowing hospitals to treat large numbers of patients in parallel rather than sequentially. In mass casualty scenarios, this enables burn units to shift into surge capacity, preserving limited surgical resources while still performing timely debridement across dozens of patients.
Building Global Capacity
The U.S. government has been a significant backer. BARDA has provided over $130 million in cumulative support, and the Department of Defense has committed approximately $18 million to develop a temperature-stable formulation suitable for battlefield use. The World Health Organization has also published guidance including enzymatic debridement as an accepted approach for burn care in mass-casualty settings.
Fire disasters capable of producing mass burn casualties are not rare. The 2023 Maui wildfires killed 102 people, with burn victims requiring airlift to Oahu. The January 2025 Los Angeles fires produced burn injuries that strained regional hospitals. Each event underscores how quickly specialized burn care capacity can be overwhelmed. NexoBrid is now approved in over 45 countries and is expanding manufacturing capacity sixfold by the end of 2025.
In the United States, commercial rights to NexoBrid are held by Vericel Corporation (NASDAQ: VCEL), reflecting the therapy’s integration into a broader burn care ecosystem across public health and commercial channels.
Beyond emergency burn care, MediWound is applying the same enzymatic approach to a far more common and persistent problem: chronic wounds. Millions of patients with diabetic foot ulcers and venous leg ulcers require debridement each year, yet the standard enzymatic treatment in this setting is still based on a drug approved more than six decades ago. These wounds are slow to heal, prone to infection, and carry a mortality risk comparable to some cancers.
EscharEx, MediWound’s late-stage investigational therapy for chronic wound debridement, is now in Phase III trials following earlier studies showing faster and more complete removal of non-viable tissue compared with the long-standing standard of care. If successful, it could reduce prolonged treatment cycles and surgical interventions in a patient population that places a growing strain on healthcare systems worldwide.
Read this Next >>
EagleNXT Announces Successful Drone and Sensor Sale to U.S. Army (UAVS)
Ironwood beats on 2026 outlook thanks to AbbVie-partnered Linzess (IRWD)
Recent News Highlights from MediWound
MediWound Reports Third Quarter 2025 Financial Results and Provides Corporate Update
MediWound Successfully Completes Commissioning of Expanded GMP Manufacturing Facility for NexoBrid
Important Disclaimers and Disclosures: The author, Wall Street Wire, is a content and media technology platform that connects the market with under-the-radar companies. The platform operates a network of industry-focused media channels spanning finance, biopharma, cyber, AI, and additional sectors, delivering insights on both broader market developments and emerging or overlooked companies. The content above is a form of paid promotional content and advertising. Wall Street Wire has received cash compensation from MediWound Ltd for promotional media services, which are provided on an ongoing basis. This content is for informational purposes only and does not constitute financial or investment advice. Wall Street Wire is not a broker-dealer or investment adviser. Full compensation details, information about the operator of Wall Street Wire, and the complete set of disclaimers and disclosures applicable to this content are available at: wallstwire.ai/disclosures. Market size figures or other estimates referenced in this article are quoted from publicly available sources; we do not independently verify or endorse them, and additional figures or estimates may exist. This article should not be considered an official communication of the issuer.